Standard Enthalpy Of Formation Quizlet . study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their.
from www.numerade.com
the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, although oxygen can exist as ozone (o 3 ), atomic. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of formation of any element in its standard state is zero by definition.
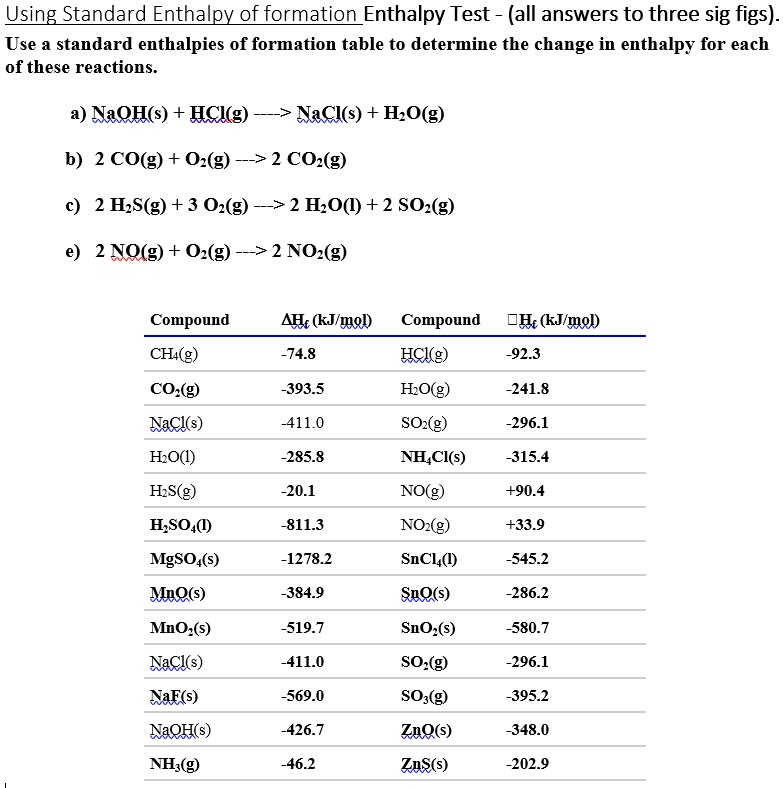
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all
Standard Enthalpy Of Formation Quizlet Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their.
From quizzschoolberg.z13.web.core.windows.net
What Is Enthalpy Quizlet Equation Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of formation is defined. Standard Enthalpy Of Formation Quizlet.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. a standard enthalpy of formation δ h f ° δ h f. Standard Enthalpy Of Formation Quizlet.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. Web. Standard Enthalpy Of Formation Quizlet.
From www.docsity.com
Standard Enthalpy of Formation (or Heat of Formation). Study notes Standard Enthalpy Of Formation Quizlet Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For. Standard Enthalpy Of Formation Quizlet.
From www.numerade.com
SOLVEDThe standard enthalpy of formation of NH3 is 46.1 kJ / mol Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. the standard enthalpy of formation, also known as the heat of formation,. Standard Enthalpy Of Formation Quizlet.
From www.studocu.com
Enthalpy definitions The Standard Enthalpy of Formation, H f, 298, is Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, although oxygen can exist as ozone (o. Standard Enthalpy Of Formation Quizlet.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Quizlet Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation Quizlet.
From www.chegg.com
Solved Using The Standard Enthalpies Of Formation Listed Standard Enthalpy Of Formation Quizlet For example, although oxygen can exist as ozone (o 3 ), atomic. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. the standard enthalpy of. Standard Enthalpy Of Formation Quizlet.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. a standard enthalpy of formation δ h. Standard Enthalpy Of Formation Quizlet.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation Quizlet For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Web. Standard Enthalpy Of Formation Quizlet.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation. Standard Enthalpy Of Formation Quizlet.
From www.numerade.com
SOLVED Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation Quizlet a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of. Standard Enthalpy Of Formation Quizlet.
From www.chegg.com
Solved Part A Use standard enthalpies of formation to Standard Enthalpy Of Formation Quizlet study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. For example, although oxygen can exist as ozone (o 3 ), atomic. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of. Standard Enthalpy Of Formation Quizlet.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Quizlet Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a. Standard Enthalpy Of Formation Quizlet.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. For example, although oxygen can exist as ozone. Standard Enthalpy Of Formation Quizlet.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Quizlet study with quizlet and memorize flashcards containing terms like standard enthalpy of formation definition, the enthalpy change. For example, although oxygen can exist as ozone (o 3 ), atomic. Δh ∘ f is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their. the standard enthalpy of. Standard Enthalpy Of Formation Quizlet.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Quizlet the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. For example, although oxygen can exist as ozone (o 3 ), atomic. a standard enthalpy of formation δ h. Standard Enthalpy Of Formation Quizlet.
From slideplayer.com
Spontanaity, Entropy, Enthalpy & Free Energy ppt download Standard Enthalpy Of Formation Quizlet a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. the standard enthalpy of formation of any element in its standard state is zero by definition. Δh ∘ f is the enthalpy change for the formation of one mole. Standard Enthalpy Of Formation Quizlet.